Dual Biomarker Assays Market Set to Triple to USD 4.3B by 2035
Global dual biomarker assays market rises as oncology and hospital diagnostics demand more accurate, multi-marker testing.
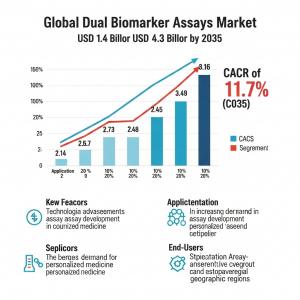
CA, UNITED STATES, November 6, 2025 /EINPresswire.com/ -- The global Dual Biomarker Assays Market, valued at USD 1.4 billion in 2025, is projected to reach USD 4.3 billion by 2035, reflecting an 11.7% compound annual growth rate over the forecast period. Growth is driven by rapid adoption of multi-marker testing in oncology, where clinicians prioritize early detection and therapy monitoring to improve patient outcomes. Cancer remains the leading clinical focus as oncologists lean toward multiplex diagnostics to enhance accuracy and reduce false negatives.
Dual biomarker assays matter now because clinicians and health systems are under pressure to increase diagnostic precision while managing cost and workflow complexity. Multi-marker detection improves sensitivity and helps avoid misclassification at early disease stages. This shift aligns with precision medicine goals, increases clinician confidence, and improves treatment matching. For suppliers, the value lies in recurring test volume, clinical integration, and tighter alignment with evolving treatment pathways.
Explore trends before investing — request a sample report today!:- https://www.futuremarketinsights.com/reports/sample/rep-gb-22362
Fast Facts
• Market Value (2025): USD 1.4B
• Forecast Value (2035): USD 4.3B
• CAGR (2025 to 2035): 11.7%
• Leading Indication (2025): Cancer, 87% share
• Leading Assay Type (2025): Immunochemistry, 60% share
• Top Growth Regions: Asia Pacific, particularly India and China
• Source or material composition share: Data not disclosed in client file.
What is winning, and why
Clinicians are choosing diagnostics that improve early-stage detection and stratify patients by therapy response likelihood. This reflects a shift from reactive to proactive care, especially in oncology.
• Product Leader: Cancer-focused dual biomarker panels (high clinical relevance and reimbursement alignment).
• Form Leader: Immunochemistry assays (automation-ready and scalable across hospital labs).
• Source Leader: Data not disclosed in client file.
Where to play
Channels
Hospital laboratories hold primary demand due to integrated patient management and established diagnostic infrastructure. Diagnostic laboratories follow, especially in markets with strong private healthcare penetration. Consumer-facing channels such as convenience stores and e-commerce are not relevant to this market; Data not disclosed in client file for any home-use distribution.
Click Here to Purchase the Report:- https://www.futuremarketinsights.com/checkout/22362
Geographic Growth Focus
• United States: CAGR 10.7%; precision oncology programs and large-scale hospital networks support stable expansion.
• Germany: CAGR 11.4%; strong molecular diagnostics reimbursement and academic partnerships drive adoption.
• Japan: CAGR 12.1%; aging population and national screening initiatives push early detection.
• China: CAGR 13.9%; government-led healthcare modernization and biotech investment accelerate uptake.
• India: CAGR 14.2%; rapid diagnostic infrastructure expansion and affordability programs increase accessibility.
What teams should do next
R&D
• Prioritize cancer-focused multiplex panels aligned with targeted therapy pathways.
• Build assay platforms compatible with automated immunochemistry systems to support hospital workflow.
• Validate dual-marker performance in early-stage detection to support clinical guideline inclusion.
Marketing & Sales
• Position around precision care outcomes, not just test accuracy.
• Equip field teams with case-based evidence demonstrating reduction of false negatives.
• Target oncology care networks and tumor board workflows for integrated adoption.
Regulatory & QA
• Align assay development to FDA/EMA companion diagnostic frameworks, especially for targeted therapies.
• Expand post-market performance surveillance to support payer and clinical confidence.
• Standardize data integration protocols for EHR and LIS connectivity to reduce lab onboarding friction.
Sourcing
• Secure stable reagent and antibody supply lines for immunochemistry platforms.
• Develop local manufacturing partnerships in India and China to address cost and access pressures.
• Invest in cold chain reliability to support transport of sensitive assay components.
Three quick plays this quarter
• Co-develop clinical education modules with oncology networks highlighting multi-marker diagnostic impact.
• Expand hospital lab integration pilots to streamline onboarding of dual biomarker workflows.
• Prioritize regulatory filing acceleration for oncology-linked companion diagnostics where targeted therapies are expanding.
The take
Dual biomarker assays are moving from specialized lab tools to clinically essential diagnostics in cancer management. The drivers are clear: hospitals want higher diagnostic confidence, oncologists need more precise patient stratification, and health systems seek earlier intervention to improve outcomes and lower long-term costs. Suppliers that support clinical workflow integration, demonstrate outcome-based value, and collaborate with oncology care ecosystems will lead. Growth depends on trust, reproducibility, and proof of clinical benefit.
Latest In-vitro Diagnostics Devices Reports:-
DNA Diagnostics Market
https://www.futuremarketinsights.com/reports/dna-diagnostics-market
Exosomes Diagnostic and Therapeutic Market
https://www.futuremarketinsights.com/reports/exosome-diagnostic-and-therapeutics-market
At-home Ulcer Testing Market
https://www.futuremarketinsights.com/reports/at-home-ulcer-testing-market
Why FMI:- https://www.futuremarketinsights.com/why-fmi
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 400 analysts worldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-347-918-3531
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.